Talk:Nerve agent
| This is the talk page for discussing improvements to the Nerve agent article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find medical sources: Source guidelines · PubMed · Cochrane · DOAJ · Gale · OpenMD · ScienceDirect · Springer · Trip · Wiley · TWL |
| Archives: Index, 1Auto-archiving period: 90 days |
| VP (nerve agent) was nominated for deletion. The discussion was closed on 6 August 2015 with a consensus to merge. Its contents were merged into Nerve agent. The original page is now a redirect to this page. For the contribution history and old versions of the redirected article, please see its history; for its talk page, see here. |
| This It is of interest to the following WikiProjects: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Daily pageviews of this article
A graph should have been displayed here but graphs are temporarily disabled. Until they are enabled again, visit the interactive graph at pageviews.wmcloud.org |
|
This page has archives. Sections older than 90 days may be automatically archived by ClueBot III when more than 5 sections are present. |
Lists of uncited chemicals[edit]
On 24 July, 79.12.242.18 added two long lists of chemicals in this series of edits. There are no citations for these chemicals. I propose to remove these lists.-- Toddy1 (talk) 15:59, 9 August 2018 (UTC)
- You have my support. These chems are not only unsourced, the text is also poorly formatted (e.g. chemically) and poorly integrated into the article. Also, just listing a bunch of chems and classifying them without telling why they are classified the way they are is not very useful info to the reader. Keministi (talk) 19:45, 9 August 2018 (UTC)
- linked (few) , from various web (chem mil etc) sources
- to shorten the lines , i t s iso ter sec , for i3 s3 mean PO O/S CH(R) (CH) X/N/CN/.. for i , s : P OS ch2 (ch) CH(R) N/X , see GV igv or v analogues
- or the structures ( C n H n N O P S F Cl Br I ... ) — Preceding unsigned comment added by 79.23.226.73 (talk) 08:52, 14 August 2018 (UTC)
Extended content
|
|---|
  The G-series (Trilon) is thus named because German scientists first synthesized them. G series agents are known as non-persistent, while the V series are persistent. All of the compounds in this class were discovered and synthesized during or prior to World War II, led by Gerhard Schrader (later under the employment of IG Farben). This series is the first and oldest family of nerve agents. The first nerve agent ever synthesised was GA (tabun) in 1936. GB (sarin) was discovered next in 1939, followed by GD (soman) in 1944, and finally the more obscure GF (cyclosarin) in 1949. GB was the only G agent that was fielded by the US as a munition, in rockets, aerial bombs, and artillery shells.[2]
R' POF OR" , POX (CN Cl) PONR2 , OR" containing N
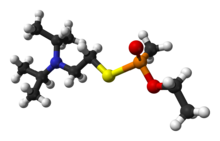  The V-series (Amiton) is the second family of nerve agents and contains five well known members: VE, VG, VM, VP (O 3 3 5 trimethyl cyclohexyl methyl phosphonate O 3-Pyridyl) , EA-3148 O cyclopentyl methyl phosphonate S ethyl diethylamine , VR, VS and VX EA1701 , along with several more obscure analogues.[3]
IVX1/2/3/4/5/6 , RO PO(R) (R isoAlkyl , iPr) or S CH(CH3)CH2NR2
trimethylamine amino > try(alkyl)ammonium , tongueslip , three - five agents with N+ Ar O (ch) o Ar O (ch) phos formulas (a quick ctrl c v search , some are on pt wiki anyway there are outhere , this group is similiar (remotely) to some carbamate agents , EAs substances include carbamates (mainly DMCa N DM) glycolates & co , else (non agents one are some metal alloys with Cu Cr Zn etc) , one : (Ph)4B- 3or5 TMA+ Ph O 1 4 Bu O (-3) (5 TMA+) Ph PO3CH3 OiPr C74H83B2N2O5P,
|
Detection section[edit]
Someone tried to add a detection section that is almost exclusively about one method used to detect nerve agents. WP:UNDUE says that "Neutrality requires that each article or other page in the mainspace fairly represent all significant viewpoints that have been published by reliable sources..." Given that there are many methods of detecting nerve agents, having a section labelled detection that is just about one of them gives a distorted and inaccurate view of the topic. The section is shown below:-- Toddy1 (talk) 14:48, 5 September 2018 (UTC)
Detection section
|
|---|
|
=== Detection === Laser photoacoustic spectroscopy (LPAS) is a method that has been used to detect nerve agents in the air. In this method, laser light is absorbed by gaseous matter. This causes a heating/cooling cycle and changes in pressure. Sensitive microphones convey sound waves that result from the pressure changes. Scientists at the U.S. Army Research Laboratory engineered an LPAS system that can detect multiple trace amounts of toxic gases in one air sample.[1] This technology contained three lasers modulated to different frequency, each producing a different sound wave tone. The different wavelengths of light were directed into a sensor referred to as the photoacoustic cell. Within the cell were the vapors of different nerve agents. The traces of each nerve agent had a signature effect on the “loudness” of the lasers’ sound wave tones.[2] Some overlap of nerve agents’ effects did occur in the acoustic results. However, it was predicted that specificity would increase as additional lasers with unique wavelengths were added.[1] Yet, too many lasers set to different wavelengths could result in overlap of absorption spectra.[2] LPAS technology can identify gases in parts per billion (ppb) concentrations.[2][3][4] The following nerve agents have been identified with this multiwavelength LPAS:[1]
Other gases and air contaminants identified with LPAS include:[5][3]
Other reported methods of detecting nerve agents are non-dispersive infrared (NDIR), traditional IR absorption and Fourier transform infrared (FTIR) spectroscopy.[3]
|
Antidote to treatment[edit]
Can't see why anyone will have a problem with this but thought I'd play it safe. I renamed the "antidote" section to "treatment" - some of the drugs mentioneddo not act as antidote (ie "a remedy to counteract the effects of poison" MW) - atropine reduces the effect a nerve agent has on the body rather than counteracting the toxin. It also mentions two separate treatments, pralidoxime and atropine, noting that they may be administered together. Standard treatment for nerve agent poisoning is to administer both (eg army issue autoinjectors such as ATNAA and Mark I NAAK contain both of these drugs), to administer one on without the other is far less effective. Everything is cited so feel free to check it out. I also added Pyridostigmine bromide to the countermeasures in development section; it is preexisting so the section needed renamed, unsure of what is appropriate so just went with "Countermeasures". Alternatively can be split into two sections; maybe one on the development of nerve agent treatments as a whole. My knowledge of this stuff isn't huge so further additions may be beyond me! Comment if you have any issues/ideas! Editor/123 17:22, 27 July 2020 (UTC) — Preceding unsigned comment added by Ben8142 (talk • contribs)
Capitalization?[edit]
Are nerve agents capitalized? FinnSoThin (talk) 00:48, 4 July 2022 (UTC)
- C-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Technology
- C-Class level-5 vital articles
- Wikipedia level-5 vital articles in Technology
- C-Class vital articles in Technology
- C-Class military history articles
- C-Class military science, technology, and theory articles
- Military science, technology, and theory task force articles
- C-Class weaponry articles
- Weaponry task force articles
- C-Class pharmacology articles
- Mid-importance pharmacology articles
- WikiProject Pharmacology articles
- C-Class medicine articles
- Mid-importance medicine articles
- C-Class toxicology articles
- High-importance toxicology articles
- Toxicology task force articles
- All WikiProject Medicine articles
- C-Class Science Policy articles
- Low-importance Science Policy articles